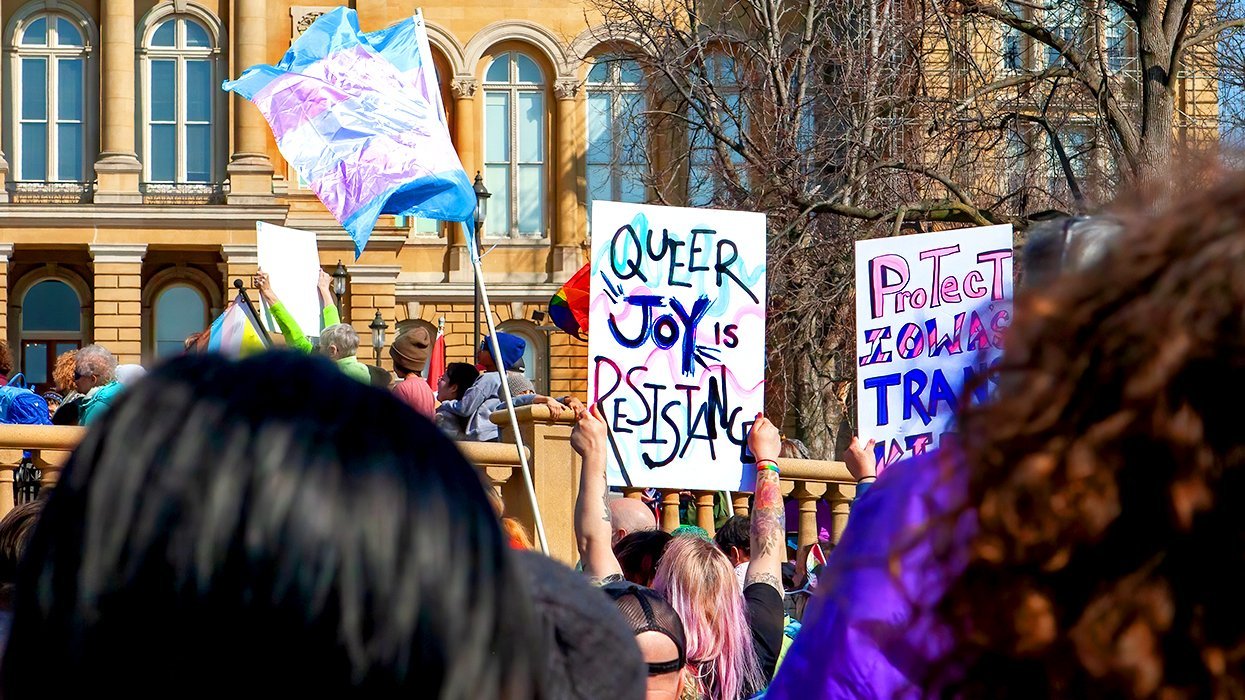CONTACTStaffCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2024 Pride Publishing Inc.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Private Policy and Terms of Use.
The Food and Drug Administration last week approved a new formulation of the protease inhibitor Invirase made by Roche. The new 500-milligram tablets will decrease the pill burden for HIV patients from 10 pills to four pills daily. The tablets also are smaller in size than the 200-milligram capsules currently available. "With the new Invirase 500-milligram tablet now available, pill burden is considerably reduced, and we anticipate that this improved convenience, together with 'boosted' Invirase's record of convincing data, will result in an expansion of its use in the earlier stages of HIV therapy," said William Burns, head of Roche's pharmaceutical division, in a press statement.
Want more breaking equality news & trending entertainment stories?
Check out our NEW 24/7 streaming service: the Advocate Channel!
Download the Advocate Channel App for your mobile phone and your favorite streaming device!
From our Sponsors
Most Popular
Here Are Our 2024 Election Predictions. Will They Come True?
November 07 2023 1:46 PM
17 Celebs Who Are Out & Proud of Their Trans & Nonbinary Kids
November 30 2023 10:41 AM
Here Are the 15 Most LGBTQ-Friendly Cities in the U.S.
November 01 2023 5:09 PM
Which State Is the Queerest? These Are the States With the Most LGBTQ+ People
December 11 2023 10:00 AM
These 27 Senate Hearing Room Gay Sex Jokes Are Truly Exquisite
December 17 2023 3:33 PM
10 Cheeky and Homoerotic Photos From Bob Mizer's Nude Films
November 18 2023 10:05 PM
42 Flaming Hot Photos From 2024's Australian Firefighters Calendar
November 10 2023 6:08 PM
These Are the 5 States With the Smallest Percentage of LGBTQ+ People
December 13 2023 9:15 AM
Here are the 15 gayest travel destinations in the world: report
March 26 2024 9:23 AM
Watch Now: Advocate Channel
Trending Stories & News
For more news and videos on advocatechannel.com, click here.
Trending Stories & News
For more news and videos on advocatechannel.com, click here.
Latest Stories
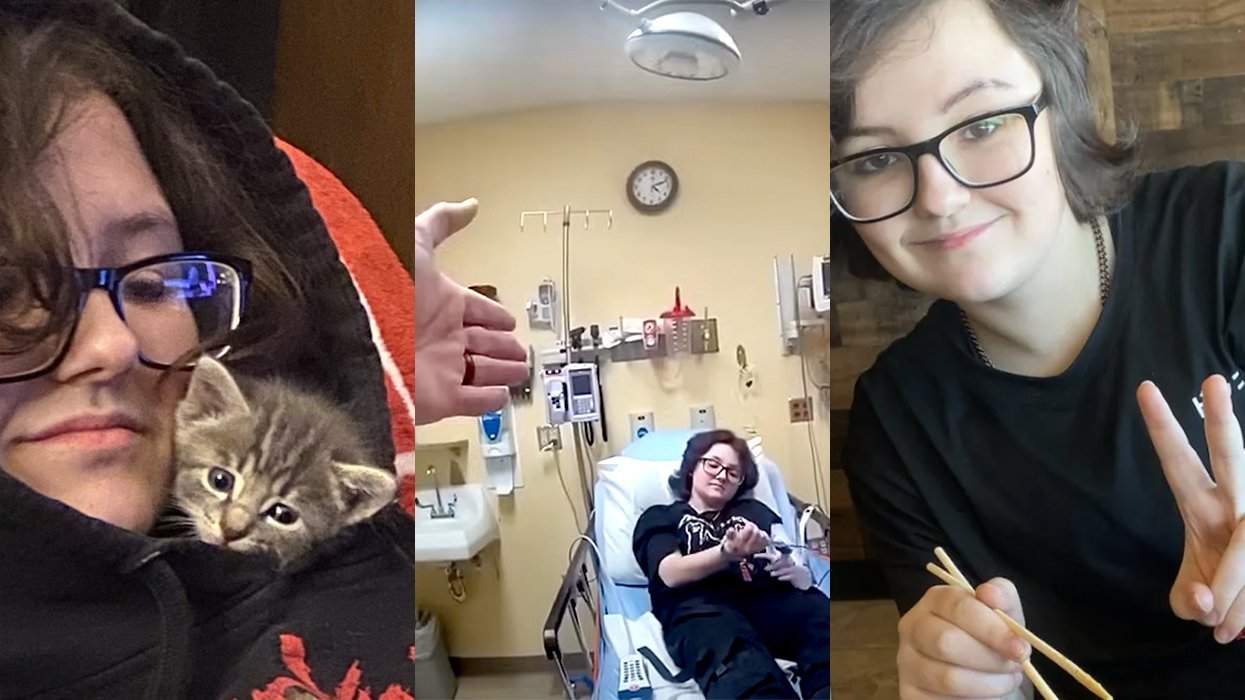
A Republican mom and her transgender son open up about unconditional love and acceptance
April 16 2024 6:00 AM
Supreme Court lets Idaho enforce law criminalizing gender-affirming care for minors
April 15 2024 8:47 PM

Plus
Yahoo FeedGay fetish artist Rex has died — see some of his sexy work
April 15 2024 8:13 PM
Brittney Griner and her wife, Cherelle, are expecting! Here's when baby Griner is arriving
April 15 2024 12:52 PM
Tennessee Senate passes bill making 'recruiting' for trans youth care a felony
April 14 2024 11:17 AM
Trending stories
Most Recent
Recommended Stories for You