CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
The Food and Drug Administration's Antiviral Drugs Advisory Committee on Thursday voted 11-3 to recommend the approval of Aptivus (tipranavir), an investigational HIV protease inhibitor developed by Boehringer Ingelheim. Aptivus is a nonpeptidic protease inhibitor that requires boosting with low-dose Norvir and must be used in combination with other antiretroviral agents. The committee's recommendations will be considered by the FDA in its review of the New Drug Application that Boehringer Ingelheim submitted for Aptivus. The FDA is not bound by the committee's recommendation but takes its advice into consideration when reviewing investigational drugs seeking approval. The FDA granted priority review status to Aptivus, which means a final marketing decision by the agency will be issued no later than August 2005. The committee's positive recommendation is based on data from two large Phase III clinical trials, conducted in protease inhibitor-resistant and treatment-experienced patients. Study subjects had taken three classes of anti-HIV drugs and were failing their protease inhibitor-based regimen at the time of study entry. The trials showed that a significant number of study subjects with resistance to other protease inhibitors responded well to Aptivus, reducing blood-based viral loads and posting improvements in CD4-cell counts.
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
Tucker Carlson and Milo Yiannopoulos spend two hours spewing homophobia and pseudo-science
December 04 2025 4:47 PM
'The Abandons' stars Gillian Anderson & Lena Headey want to make lesbian fans proud
December 04 2025 4:38 PM
Tig Notaro is working on a 'hot lesbian action' movie with Zack Snyder
December 04 2025 4:36 PM
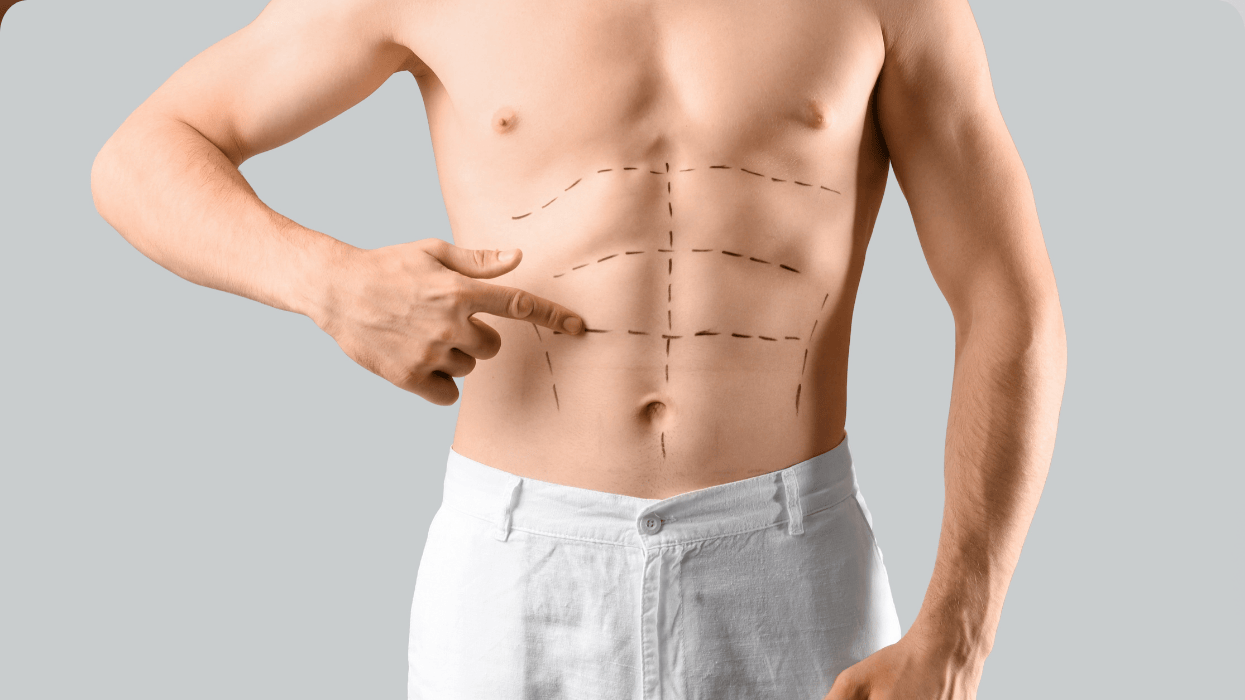
Cis men love top surgery—it should be available for all
December 04 2025 4:35 PM
Denver LGBTQ+ youth center closed indefinitely after burglar steals nearly $10K
December 04 2025 12:57 PM
Trans pastor says she’s ‘surrounded by loving kindness’ after coming out to New York congregation
December 04 2025 11:13 AM
Lesbian educator wins $700K after she was allegedly called a ‘witch’ in an ‘LGBTQ coven’
December 04 2025 10:59 AM
Years before Stonewall, a cafeteria riot became a breakthrough for trans rights
December 04 2025 10:50 AM
Charlie Kirk’s widow set to join out CBS News chief Bari Weiss for televised town hall
December 04 2025 10:20 AM
Women's Institute to ban transgender women after U.K. Supreme Court ruling
December 03 2025 4:10 PM
Trending stories
Recommended Stories for You



































































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes