CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
The Food and Drug Administration has granted fast-track review status to the experimental HIV fusion inhibitor Fuzeon, formerly known as T-20, a move that could make the drug available to the public by mid March. Approval could have taken twice as long without the fast-track review status. The FDA will review the drug no later than March 16. Drugmakers Roche and Trimeris, which jointly developed Fuzeon, filed for FDA review in September. Fuzeon is the first drug in a new class of HIV antiretrovirals that aim to prevent the virus from being able to attach to and infect immune system cells. The FDA application for the drug seeks approval for patients who are not responding to other anti-HIV medications, but studies have shown that Fuzeon may be even more effective in treating people recently infected with the virus. The drug, which must be injected, is expected to cost between $12,000 and $15,000 per year
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Gay makeup artist Andry Hernández Romero describes horrific sexual & physical abuse at CECOT in El Salvador
July 24 2025 10:11 AM
True
Watch Now: Pride Today
Latest Stories
Missing Black trans man Danny Siplin found dead in Rochester, New York
December 29 2025 8:45 PM
'Heated Rivalry' season 2: every steamy & romantic moment from the book we can't wait to see
December 29 2025 5:27 PM
Chappell Roan apologizes for praising late Brigitte Bardot: 'very disappointing'
December 29 2025 4:30 PM
RFK Jr.'s HHS investigates Seattle Children's Hospital over youth gender-affirming care
December 29 2025 1:00 PM
Zohran Mamdani claps back after Elon Musk attacks out lesbian FDNY commissioner appointee
December 29 2025 11:42 AM
Trump's gay Kennedy Center president demands $1M from performer who canceled Christmas Eve show
December 29 2025 10:09 AM
What does 2026 have in store for queer folks? Here’s what's written in the stars
December 29 2025 9:00 AM
In 2025, being trans in America means living under conditional citizenship
December 29 2025 6:00 AM
Here are the best shows on and off-Broadway of 2025
December 26 2025 7:00 AM
10 of the sexiest music videos that gagged everyone in 2025
December 25 2025 9:30 AM
Far-right, anti-LGBTQ+ Project 2025 will continue into 2026
December 24 2025 6:34 PM
Democratic officials sue RFK Jr. over attempt to limit gender-affirming care for trans youth
December 24 2025 4:30 PM
Heated Rivalry season 2: Everything we know so far
December 24 2025 3:30 PM
Who is Lillian Bonsignore — set to be first out gay Fire Department of New York commissioner?
December 23 2025 6:21 PM
True
The HIV response on a cliff-edge: advocacy must drive urgent action to end the epidemic
December 23 2025 2:23 PM



















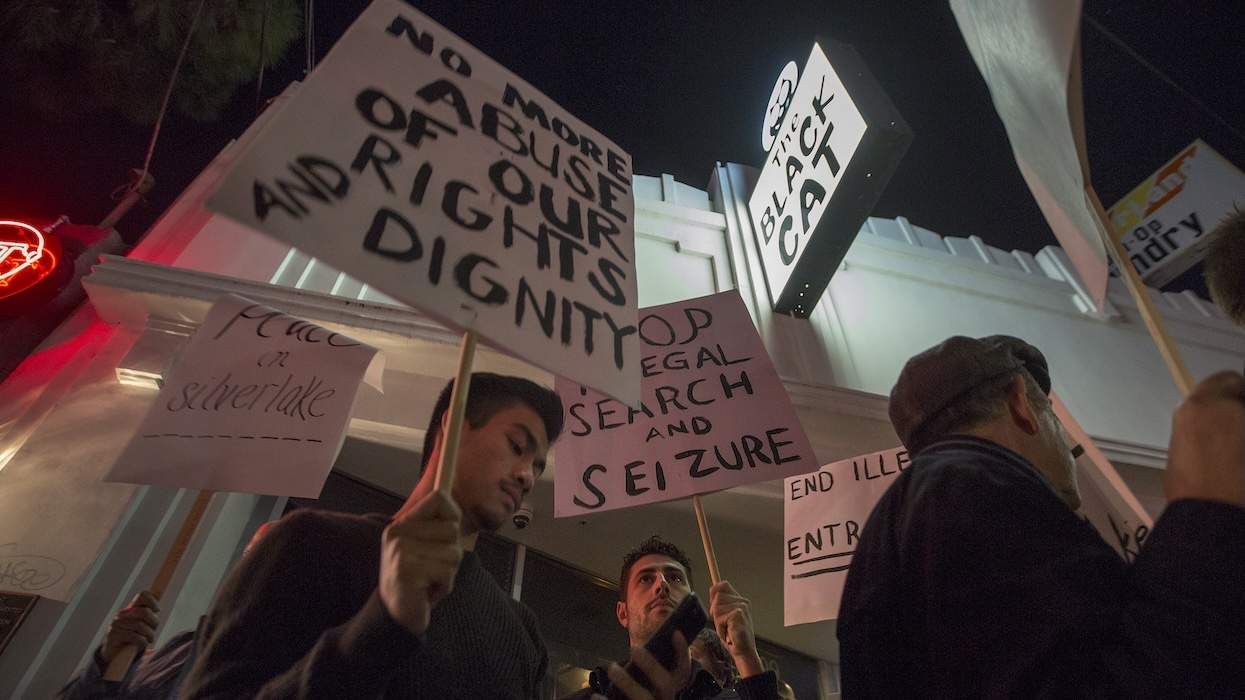


















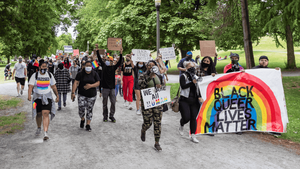




























Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes