CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Chiron Corp. announced Wednesday that it is ending the development of three compounds, including a recombinant form of interleukin-2 being studied in a Phase III trial for the treatment of patients with HIV. The company had been expected to complete the clinical trial, including patient follow-up, in 2007. Chiron is now reviewing data for a possible regulatory submission for approval of IL-2 only in a small subset of HIV-positive patients who do not receive any response to other anti-HIV medications. The company also stopped development of compounds intended to treat cystic fibrosis and hepatitis B.
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
Trump's FDA sends warning letters to companies selling chest binders
December 19 2025 2:31 PM
Bowen Yang to leave SNL after Ariana Grande and Cher episode
December 19 2025 2:10 PM
Notorious anti-LGBTQ+ New York Archbishop Dolan retires — here are his worst moments
December 19 2025 1:27 PM
Sarah McBride knew some Democrats would betray trans people, so she lobbied Republicans
December 19 2025 12:55 PM
Creating Change Returns to Washington D.C. for 38th Convening for LGBTQ Advocacy
December 19 2025 12:22 PM
House passes bill banning Medicaid from covering gender-affirming care for youth
December 19 2025 11:05 AM
Health policy expert to RFK Jr.: You can't ban trans youth care this way
December 18 2025 5:37 PM
12 lesbian thrillers and mysteries to binge & where to watch them
December 18 2025 4:36 PM
Netflix's 'Boots' season 2 plot revealed by producer amid cancelation
December 18 2025 4:33 PM
Charlie Kirk's accused killer, Tyler Robinson, on LGBTQ+ issues: It's complicated
December 18 2025 4:04 PM
Sacramento man still in coma six weeks after suspected anti-LGBTQ+ hate crime
December 18 2025 1:17 PM
RFK Jr. and Dr. Oz announce sweeping measures to ban gender-affirming care for trans youth
December 18 2025 12:19 PM
True
Texas city will remove rainbow crosswalks under orders from Trump administration
December 18 2025 11:07 AM
Six key takeaways from Trump's speech to the nation, including 'transgender for everybody'
December 17 2025 10:51 PM
Marjorie Taylor Greene’s bill criminalizing gender-affirming care for minors passes with Democrats’ support
December 17 2025 6:47 PM
True
I didn’t just run the world’s major marathons. I changed them
December 17 2025 4:31 PM
Pam Bondi wants FBI to offer bounties for ‘radical gender ideology’ groups, leaked memo shows
December 17 2025 3:17 PM












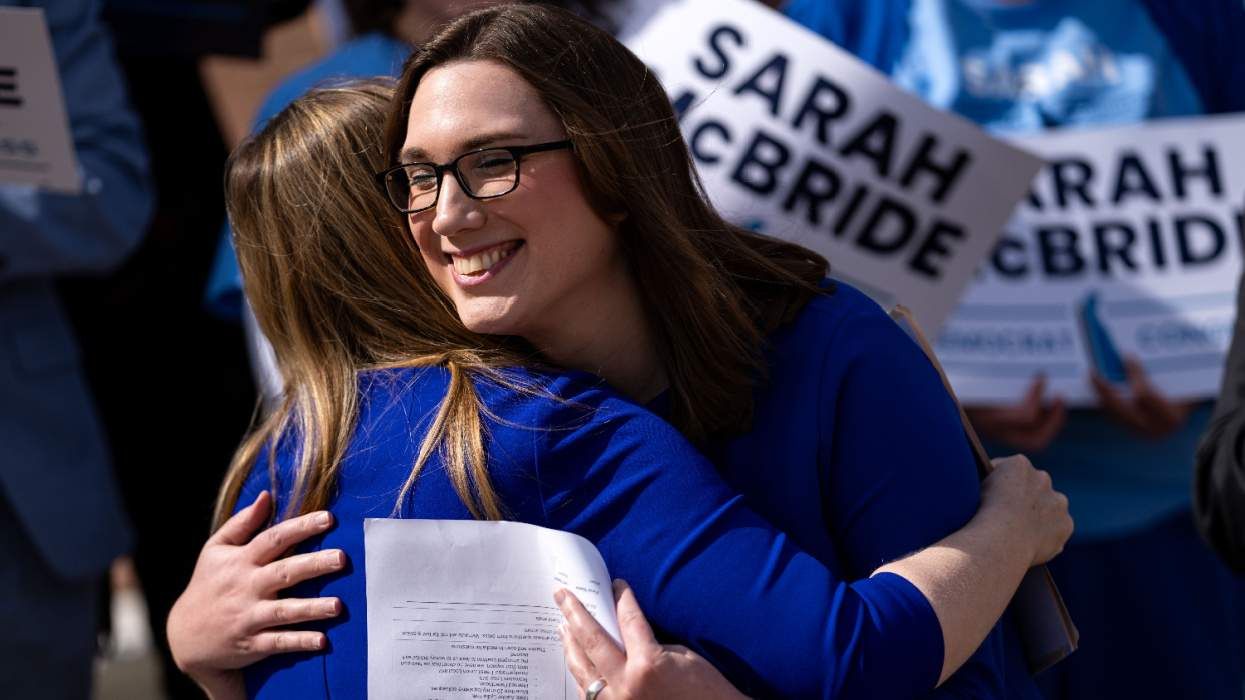

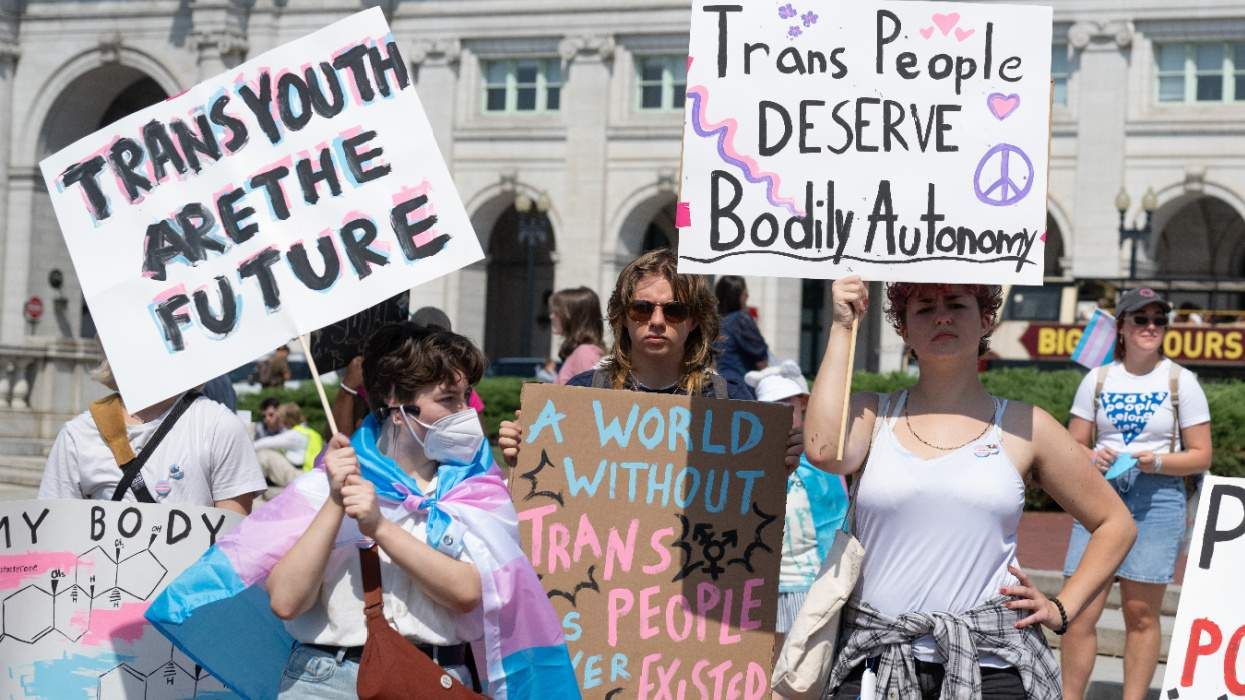




















































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes