CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
An experimental HIV drug from Merck & Co. Inc. should be quickly approved for use by patients running out of treatment options, federal advisers recommended Wednesday.
The panel of outside experts agreed unanimously that available data support accelerated approval of Isentress, also known as raltegravir, by the Food and Drug Administration.
The FDA isn't required to follow the advice of its outside advisory panels but does so most of the time. Merck expects an agency decision by mid-October. If approved, Isentress would be the first in a new class of antiretroviral drugs called integrase inhibitors.
The Merck drug targets integrase, one of three enzymes used by the virus to replicate and infect cells. The FDA previously has approved drugs that target the two other enzymes, protease and reverse transcriptase.
Isentress is meant to be used as part of a ''cocktail'' of drugs to fight HIV in patients who have developed a resistance to older medications.
Last month, the FDA approved another novel HIV drug, Pfizer Inc.'s Selzentry. That drug is the first that works by blocking a crucial doorway, called the CCR5 receptor, that HIV often uses to enter white blood cells. (AP)
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Gay makeup artist Andry Hernández Romero describes horrific sexual & physical abuse at CECOT in El Salvador
July 24 2025 10:11 AM
True
Watch Now: Pride Today
Latest Stories
A heart filled with trans hate is how Marjorie Taylor Greene is choosing to be remembered
December 20 2025 10:00 AM
Trump's FDA sends warning letters to companies selling chest binders
December 19 2025 2:31 PM
Bowen Yang to leave SNL after Ariana Grande and Cher episode
December 19 2025 2:10 PM
Notorious anti-LGBTQ+ New York Archbishop Dolan retires — here are his worst moments
December 19 2025 1:27 PM
Sarah McBride knew some Democrats would betray trans people, so she lobbied Republicans
December 19 2025 12:55 PM
Creating Change Returns to Washington D.C. for 38th Convening for LGBTQ Advocacy
December 19 2025 12:22 PM
House passes bill banning Medicaid from covering gender-affirming care for youth
December 19 2025 11:05 AM
Health policy expert to RFK Jr.: You can't ban trans youth care this way
December 18 2025 5:37 PM
12 lesbian thrillers and mysteries to binge & where to watch them
December 18 2025 4:36 PM
Netflix's 'Boots' season 2 plot revealed by producer amid cancelation
December 18 2025 4:33 PM
Charlie Kirk's accused killer, Tyler Robinson, on LGBTQ+ issues: It's complicated
December 18 2025 4:04 PM
Sacramento man still in coma six weeks after suspected anti-LGBTQ+ hate crime
December 18 2025 1:17 PM
RFK Jr. and Dr. Oz announce sweeping measures to ban gender-affirming care for trans youth
December 18 2025 12:19 PM
True
Texas city will remove rainbow crosswalks under orders from Trump administration
December 18 2025 11:07 AM
Six key takeaways from Trump's speech to the nation, including 'transgender for everybody'
December 17 2025 10:51 PM
Marjorie Taylor Greene’s bill criminalizing gender-affirming care for minors passes with Democrats’ support
December 17 2025 6:47 PM
True
I didn’t just run the world’s major marathons. I changed them
December 17 2025 4:31 PM
Pam Bondi wants FBI to offer bounties for ‘radical gender ideology’ groups, leaked memo shows
December 17 2025 3:17 PM
Trending stories
Recommended Stories for You













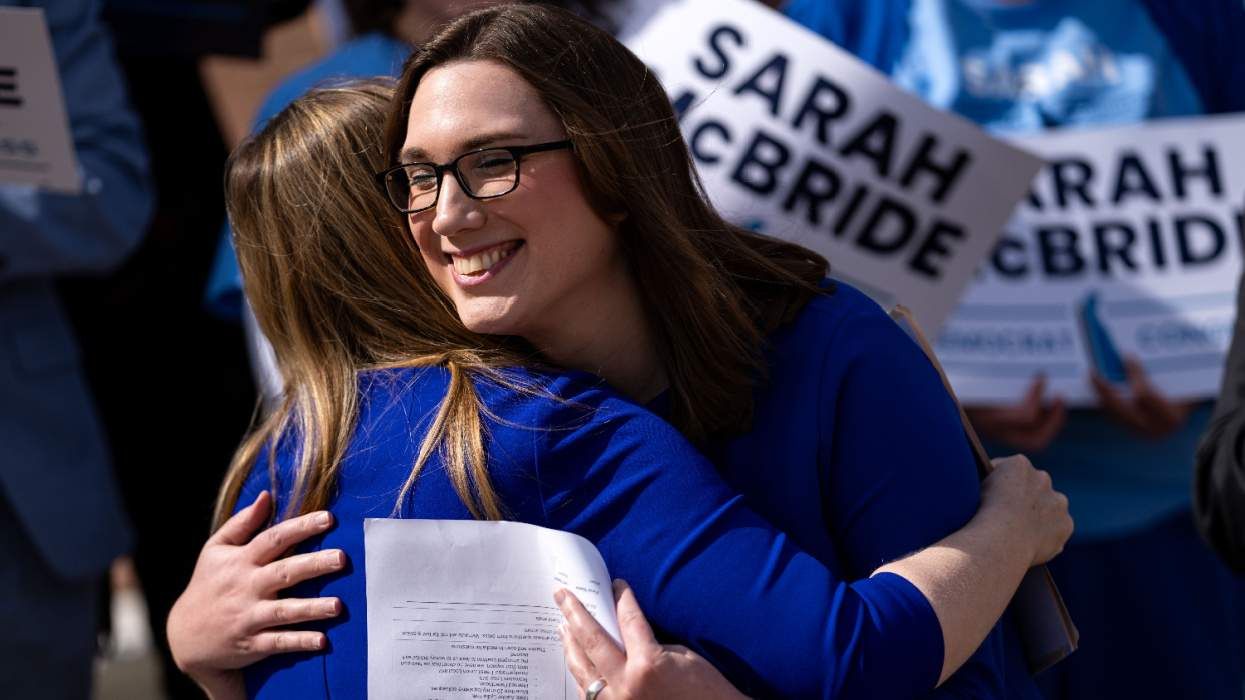




















































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes