June 16 2006 3:32 PM EST
CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
GlaxoSmithKline said on Thursday that U.S. regulators have approved the use of its drug Hycamtin in combination with cisplatin for the treatment of cervical cancer that can't be treated with surgery or radiation therapy. The Food and Drug Administration approval is based on late-stage results that demonstrated a survival advantage by using Hycamtin in combination with cisplatin compared with cisplatin alone. The company is currently developing a vaccine for the human papilloma virus, the leading cause of cervical cancer. Hycamtin is currently marketed in the United States for the treatment of a type of lung cancer. (Reuters)
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
California hospital will continue youth gender-affirming care after families protest
December 17 2025 11:18 AM
Tennessee whistleblower says library board chair sought private data as part of state's book purge
December 17 2025 7:00 AM
Lesbian federal worker pleads for answers about wife trapped in immigration detention limbo
December 16 2025 5:08 PM
Michigan Republican U.S. Senate candidate Mike Rogers surrounds himself with hardcore LGBTQ+ rights opponents
December 16 2025 2:53 PM
True
Florida city installs Pride bike racks after being forced to remove rainbow crosswalks
December 16 2025 2:21 PM
Ariana Grande and Jonathan Bailey in talks to star in West End musical
December 16 2025 12:26 PM
Netflix's 'Boots' is canceled: Stars react to the heartbreaking news
December 16 2025 11:37 AM
How this Minnesota city redefined LGBTQ+ rights 50 years ago
December 16 2025 11:25 AM
Gen Z women are more likely to identify as bisexual but still embrace lesbian label: study
December 16 2025 11:10 AM
Is Texas using driver's license data to track transgender residents?
December 15 2025 6:46 PM
Trending stories
Recommended Stories for You






















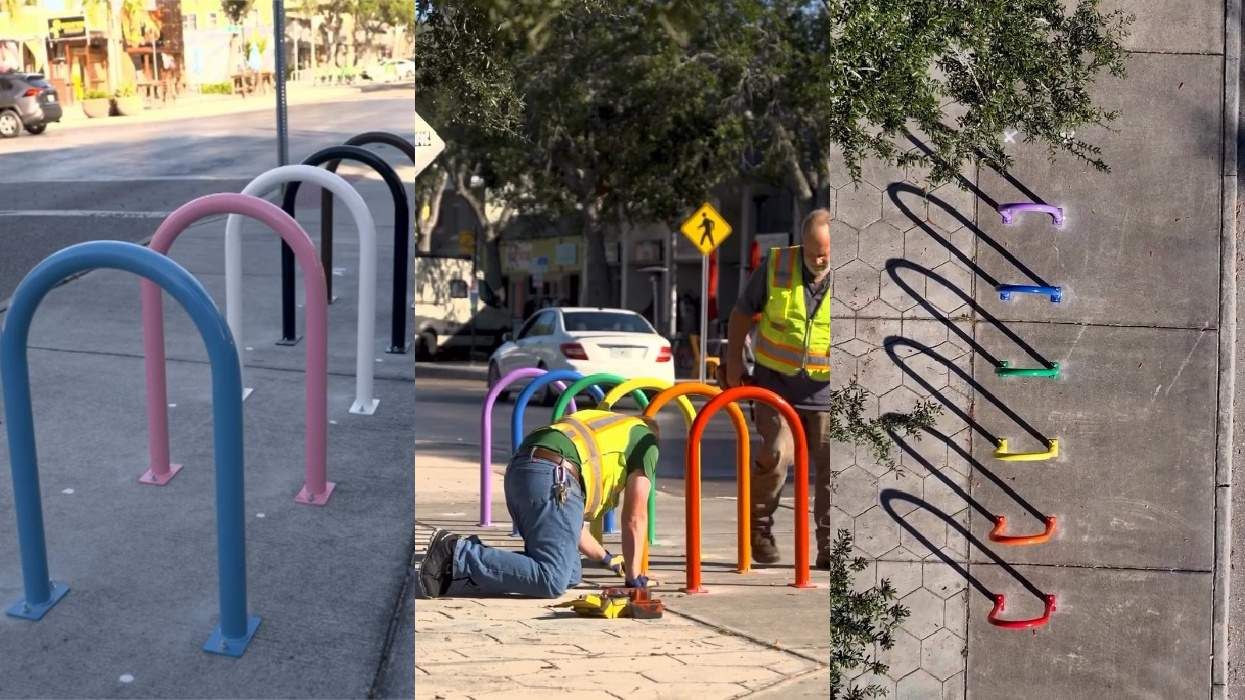











































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes