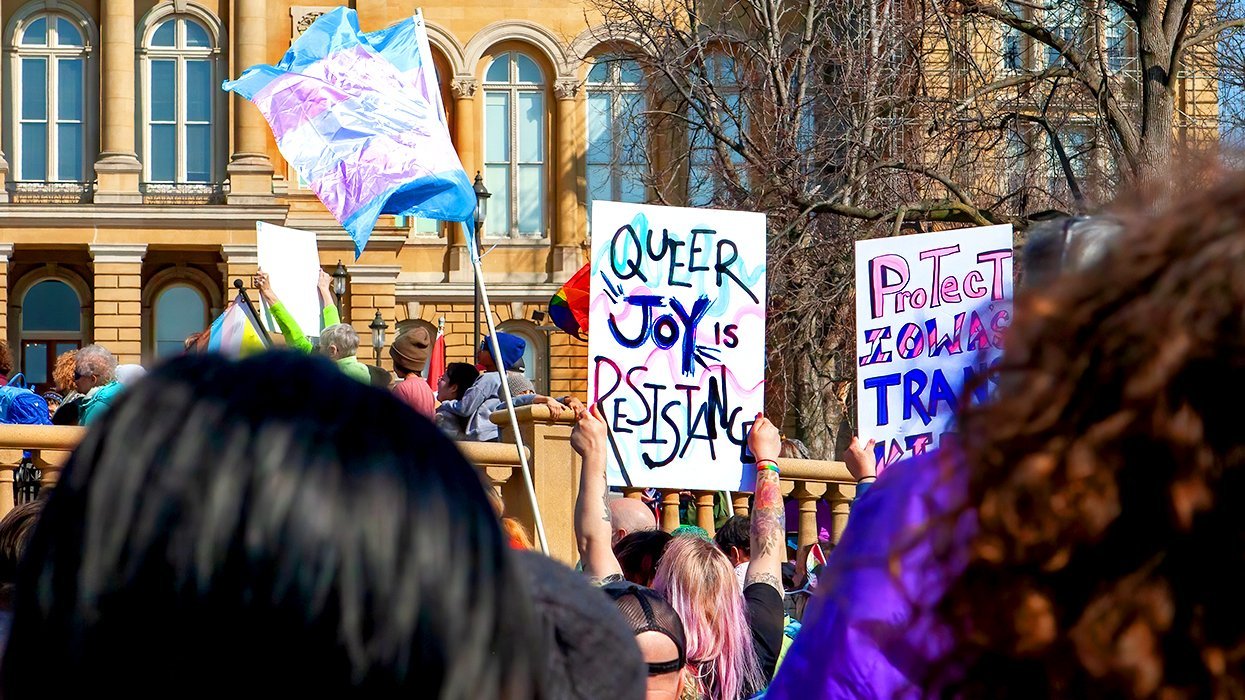CONTACTStaffCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2024 Pride Publishing Inc.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Private Policy and Terms of Use.
The Food and Drug Administration announced Monday that it has granted fast-track review status to VaxGen's experimental HIV vaccine AIDSVAX, currently wrapping up two Phase III clinical trials. The vaccine is being studied in the United States among men who have sex with men and in Thailand among injection-drug users. Nearly 8,000 people are participating in the clinical trials. VaxGen officials say the trials will be completed before the end of the year and that the company will have completed an examination of the trial data by the end of the first quarter of 2003. "Once we have the data, we can seek a six-month priority review," said VaxGen spokesman Jim Key. If trial results are successful, AIDSVAX could be available for use by 2005. Early data has shown that the vaccine does convey some protection against HIV infection but that it is not 100% effective. Industry analysts have said that because of this, the vaccine will be useful only for groups at high risk for HIV infection, including residents of developing countries.
Want more breaking equality news & trending entertainment stories?
Check out our NEW 24/7 streaming service: the Advocate Channel!
Download the Advocate Channel App for your mobile phone and your favorite streaming device!
From our Sponsors
Most Popular
Here Are Our 2024 Election Predictions. Will They Come True?
November 07 2023 1:46 PM
17 Celebs Who Are Out & Proud of Their Trans & Nonbinary Kids
November 30 2023 10:41 AM
Here Are the 15 Most LGBTQ-Friendly Cities in the U.S.
November 01 2023 5:09 PM
Which State Is the Queerest? These Are the States With the Most LGBTQ+ People
December 11 2023 10:00 AM
These 27 Senate Hearing Room Gay Sex Jokes Are Truly Exquisite
December 17 2023 3:33 PM
10 Cheeky and Homoerotic Photos From Bob Mizer's Nude Films
November 18 2023 10:05 PM
42 Flaming Hot Photos From 2024's Australian Firefighters Calendar
November 10 2023 6:08 PM
These Are the 5 States With the Smallest Percentage of LGBTQ+ People
December 13 2023 9:15 AM
Here are the 15 gayest travel destinations in the world: report
March 26 2024 9:23 AM
Watch Now: Advocate Channel
Trending Stories & News
For more news and videos on advocatechannel.com, click here.
Trending Stories & News
For more news and videos on advocatechannel.com, click here.
Latest Stories
Brittney Griner and her wife Cherelle are expecting! Here's when baby Griner is arriving
April 15 2024 12:52 PM
Tennessee Senate passes bill making 'recruiting' for trans youth care a felony
April 14 2024 11:17 AM
Italy’s prime minister says surrogacy ‘inhuman’ as party backs steeper penalties
April 14 2024 10:36 AM
After decades of silent protest, advocates and students speak out for LGBTQ+ rights
April 13 2024 10:52 AM
11 celebs who love their LGBTQ+ siblings
April 13 2024 10:33 AM
The 10 most challenged books of last year
April 13 2024 10:06 AM
Mary & George's Julianne Moore on Mary's sexual fluidity and queer relationship
April 13 2024 10:00 AM
Investigation launched after man screams homophobic slurs at queer couples on D.C. metro
April 13 2024 9:59 AM