CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
The Food and Drug Administration on Wednesday approved Hoffman-La Roche's hepatitis C treatment Pegasys, a long-lasting form of interferon, The Washington Post reports. Current hepatitis C treatments include a combination regimen of interferon and ribavirin, with interferon being injected three times per week. Pegasys is injected only once per week. Clinical studies showed that Pegasys, combined with ribavirin, is more effective at lowering hepatitis C viral levels than the standard interferon-ribavirin treatment. Roche is currently seeking FDA approval of its ribavirin drug, Copegus. The company hopes to begin marketing a combination regimen of Pegasys and Copegus in the United States early next year.
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
Texas expands lawsuits against doctors accused of providing gender-affirming care to youth
December 11 2025 4:36 PM
How Sundance 2026 celebrates its queer legacy
December 11 2025 3:54 PM
George Santos’s exclusive D.C. Christmas party featured famous grifters & MAGA influence peddlers
December 11 2025 3:31 PM
Nancy Mace investigated for bad behavior at airport, blames transgender people
December 11 2025 1:11 PM
Pete Buttigieg mocks Trump Transportation Secretary Sean Duffy’s strange airport pull-up stunt
December 11 2025 1:00 PM
Appeals court mulls upholding ruling that struck down Pentagon’s HIV enlistment ban
December 11 2025 11:51 AM
Florida sues leading medical groups for supporting gender-affirming care
December 11 2025 11:02 AM
Behind Marjorie Taylor Greene's latest push to criminalize gender-affirming care
December 10 2025 9:09 PM
Queer actor Wenne Alton Davis, known for 'Maisel,' 'Normal Heart,' killed in NYC car crash
December 10 2025 5:14 PM
‘Proud’ pro-LGBTQ+ Democrat flips Republican state House seat in Georgia electoral upset
December 10 2025 4:05 PM
Texas city votes to overturn LGBTQ+ antidiscrimination protections
December 10 2025 4:03 PM
Pornhub's spicy stats prove just how horny 2025 was
December 10 2025 3:30 PM
'Heated Rivalry' stars thank WeHo gay bar for 'tweeting about our butts'
December 10 2025 2:55 PM









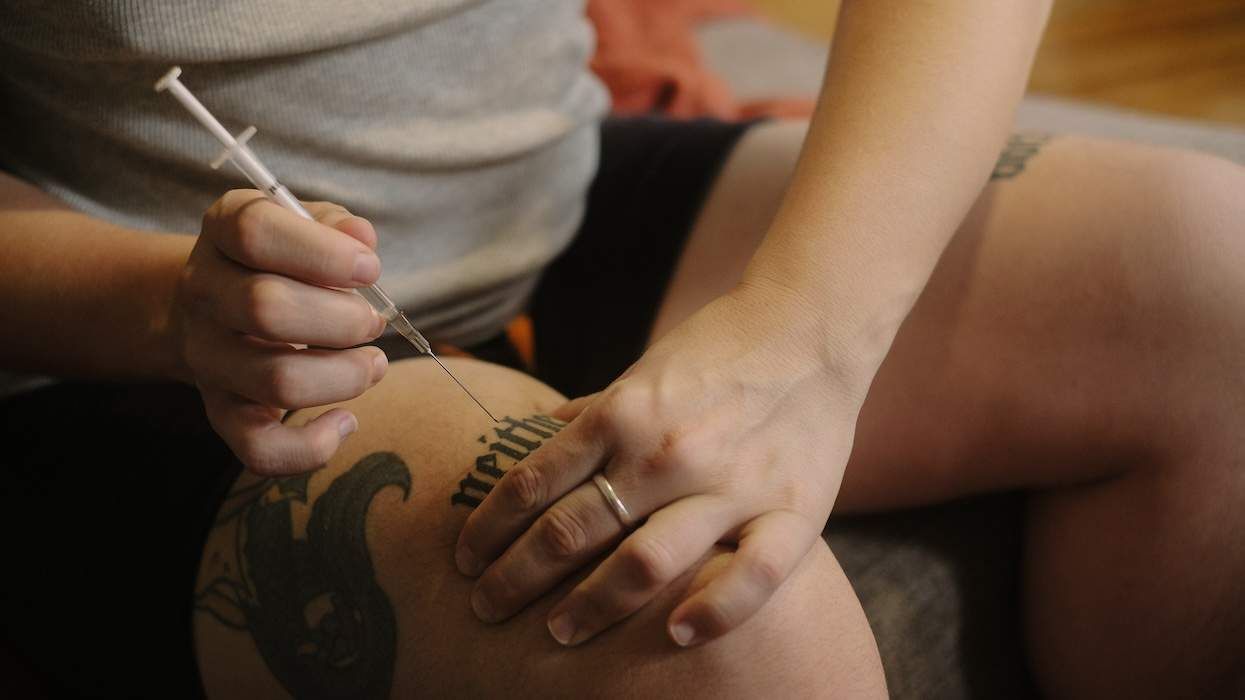

























































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes