CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
New FDA administrator Mark McClellan last week outlined his top goals for the federal agency, which include speeding market entry of safe medicines, hastening marketing of generic drugs, updating drug manufacturing standards, retaining FDA scientists, and decreasing medical errors, Dow Jones Business News reports. McClellan, who was confirmed by the Senate last month as FDA head but has not yet officially taken over, was speaking at a November 6 conference in Washington, D.C. Pharmaceutical companies have said that the FDA drug review process has delayed the launch of products on the U.S. market, which they say has led them to submit fewer FDA new drug applications. For example, in fiscal 2002, the FDA accepted 21 new product applications, down from 44 in 1997. The average review period for a new product at the FDA currently averages about 15 months. "There are, I'm sure, a number of reasons responsible for the decline in [new drug] applications in recent years, and one of the things we can do to help address that is to make sure our approval process is working as efficiently as possible," McClellan said.
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
There’s a testosterone crisis, the FDA says — for cisgender men
December 12 2025 4:59 PM
Budapest mayor could face charges for hosting LGBTQ+ Pride march
December 12 2025 4:13 PM
Jason Collins, first out gay NBA player, reveals he has 'deadliest form of brain cancer'
December 12 2025 2:09 PM
The Democratic candidate in the Texas Senate race is going to be an LGBTQ+ ally
December 12 2025 12:55 PM
Texas expands lawsuits against doctors accused of providing gender-affirming care to youth
December 11 2025 4:36 PM
How Sundance 2026 celebrates its queer legacy
December 11 2025 3:54 PM
George Santos’s exclusive D.C. Christmas party featured famous grifters & MAGA influence peddlers
December 11 2025 3:31 PM
Nancy Mace investigated for bad behavior at airport, blames transgender people
December 11 2025 1:11 PM
Pete Buttigieg mocks Trump Transportation Secretary Sean Duffy’s strange airport pull-up stunt
December 11 2025 1:00 PM
Appeals court mulls upholding ruling that struck down Pentagon’s HIV enlistment ban
December 11 2025 11:51 AM
Florida sues leading medical groups for supporting gender-affirming care
December 11 2025 11:02 AM
Behind Marjorie Taylor Greene's latest push to criminalize gender-affirming care
December 10 2025 9:09 PM
Queer actor Wenne Alton Davis, known for 'Maisel,' 'Normal Heart,' killed in NYC car crash
December 10 2025 5:14 PM
‘Proud’ pro-LGBTQ+ Democrat flips Republican state House seat in Georgia electoral upset
December 10 2025 4:05 PM
Texas city votes to overturn LGBTQ+ antidiscrimination protections
December 10 2025 4:03 PM
Pornhub's spicy stats prove just how horny 2025 was
December 10 2025 3:30 PM
'Heated Rivalry' stars thank WeHo gay bar for 'tweeting about our butts'
December 10 2025 2:55 PM









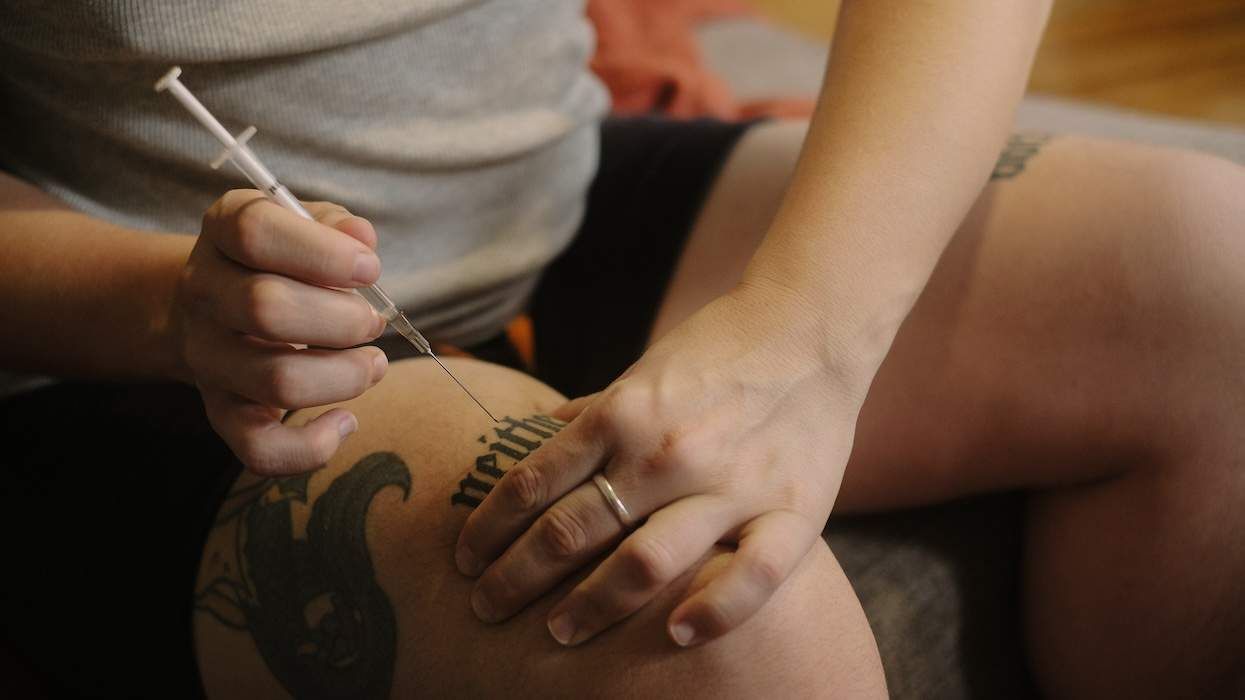

























































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes