CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
The Food and Drug Administration will establish a new independent Drug Safety Oversight Board to monitor FDA-approved medicines--including HIV antiretroviral drugs and treatments for the adverse side effects of anti-HIV drugs--once they're on the market and update physicians and patients with emerging information on risks and benefits. House and Human Services secretary Mike Leavitt announced the creation of the board during a meeting with FDA employees Tuesday. The agency has been criticized sharply in recent months as reacting too slowly to reports linking the arthritis drug Vioxx and pain drug Celebrex to increased risks of heart attack and stroke. Leavitt said it's clear that people want more oversight and openness from the agency. "They want to know what we know, what we do with information, and why we do it," he said, promising to create "a new culture of openness and enhanced independence." The board will recommend what information and updates to put on the government's Drug Watch, resolve disputes over drug safety issues, and oversee the development of a drug safety policy. It will be composed of FDA employees and medical experts from other HHS agencies and governmental departments and consult with outside medical experts as well as consumer and patient groups, officials said. To improve new drug safety information reaching patients and doctors, the board will create a drug safety Web page with emerging information--such as side effects, safety risks, and steps that can be taken to minimize them--for both previously and newly improved drugs. Also, separate information sheets for health care professionals and patients will be made widely available, officials said. (AP)
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
There’s a testosterone crisis, the FDA says — for cisgender men
December 12 2025 4:59 PM
Budapest mayor could face charges for hosting LGBTQ+ Pride march
December 12 2025 4:13 PM
Jason Collins, first out gay NBA player, reveals he has 'deadliest form of brain cancer'
December 12 2025 2:09 PM
The Democratic candidate in the Texas Senate race is going to be an LGBTQ+ ally
December 12 2025 12:55 PM
Texas expands lawsuits against doctors accused of providing gender-affirming care to youth
December 11 2025 4:36 PM
How Sundance 2026 celebrates its queer legacy
December 11 2025 3:54 PM
George Santos’s exclusive D.C. Christmas party featured famous grifters & MAGA influence peddlers
December 11 2025 3:31 PM
Nancy Mace investigated for bad behavior at airport, blames transgender people
December 11 2025 1:11 PM
Pete Buttigieg mocks Trump Transportation Secretary Sean Duffy’s strange airport pull-up stunt
December 11 2025 1:00 PM
Appeals court mulls upholding ruling that struck down Pentagon’s HIV enlistment ban
December 11 2025 11:51 AM
Florida sues leading medical groups for supporting gender-affirming care
December 11 2025 11:02 AM
Behind Marjorie Taylor Greene's latest push to criminalize gender-affirming care
December 10 2025 9:09 PM
Queer actor Wenne Alton Davis, known for 'Maisel,' 'Normal Heart,' killed in NYC car crash
December 10 2025 5:14 PM
‘Proud’ pro-LGBTQ+ Democrat flips Republican state House seat in Georgia electoral upset
December 10 2025 4:05 PM
Texas city votes to overturn LGBTQ+ antidiscrimination protections
December 10 2025 4:03 PM
Pornhub's spicy stats prove just how horny 2025 was
December 10 2025 3:30 PM
'Heated Rivalry' stars thank WeHo gay bar for 'tweeting about our butts'
December 10 2025 2:55 PM
Trending stories
Recommended Stories for You










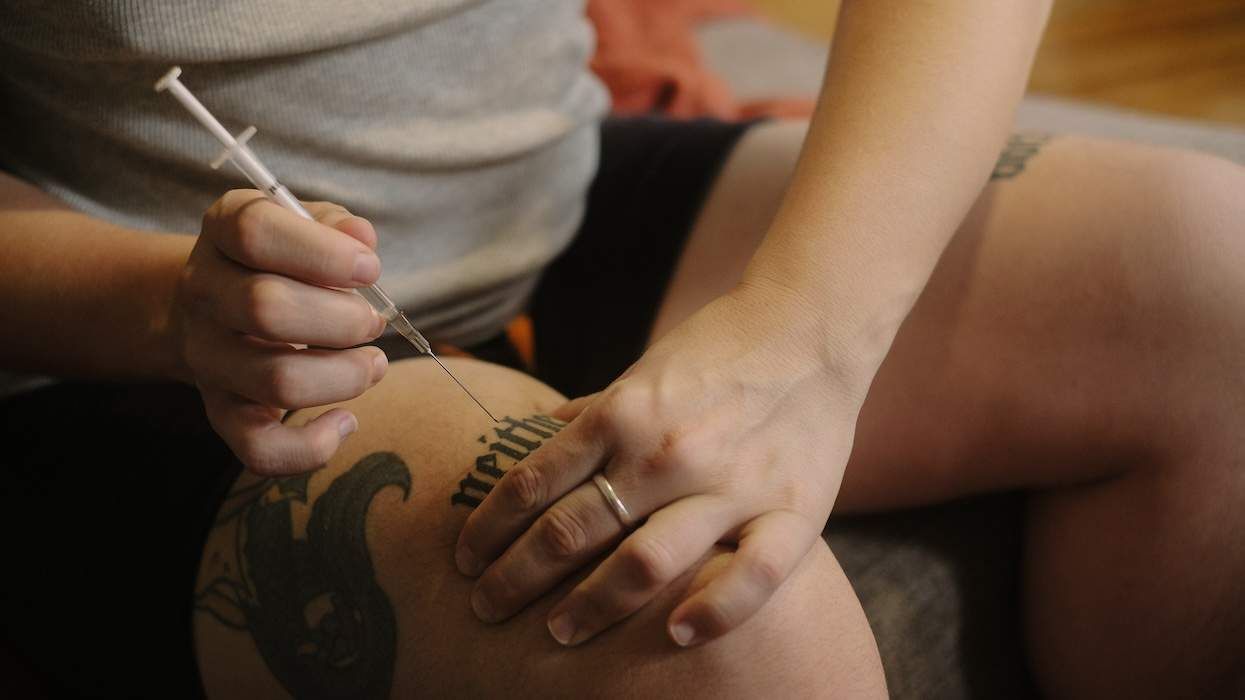

























































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes