CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
The Food and Drug Administration on Wednesday approved Boehringer Ingelheim's nonpeptidic protease inhibitor Aptivus (tipranavir) to be used in combination with other antiretroviral drugs to treat HIV infection. BI officials say the drug will be available at pharmacies nationwide within the next two weeks. The company hopes to gain approval to market the drug in Europe by the end of the year. Because Aptivus is not a peptide-based drug and has a different chemical structure than other protease inhibitors, it has been shown to be effective against strains of the virus that have developed defenses against other anti-HIV drugs. The drug is approved for HIV patients who have failed other anti-HIV regimens or are resistant to other protease inhibitors; it is not approved for first-line therapy, according to AIDSmeds.com. The U.S. Department of Health and Human Services has not yet developed guidelines for use of Aptivus among U.S. HIV patients. The recommended dosage for Aptivus is two 250-milligram capsules taken twice daily. The drug also must be taken with a small booster dose of Abbott Laboratories' protease inhibitor Norvir for maximum effect. An FDA advisory panel last month supported Aptivus's approval but called on BI to conduct long-term studies to evaluate the effects of Aptivus on blood-based lipid levels and liver function. The drug was shown in clinical trials to cause liver damage and hepatitis-like symptoms in some patients taking it. BI currently recommends that doctors give liver function tests to all HIV patients beginning Aptivus therapy and carefully monitor them throughout treatment. Extra caution is recommended for HIV patients also coinfected with hepatitis B or C who begin the drug. BI is currently conducting Phase II and III clinical trials of Aptivus involving HIV-positive children and adults who have not taken other anti-HIV drugs. The FDA has approved eight other protease inhibitor medications since 1996. The drugs work by blocking the action of an enzyme that cuts HIV proteins into smaller sections that the virus needs to replicate.
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
There’s a testosterone crisis, the FDA says — for cisgender men
December 12 2025 4:59 PM
Budapest mayor could face charges for hosting LGBTQ+ Pride march
December 12 2025 4:13 PM
Jason Collins, first out gay NBA player, reveals he has 'deadliest form of brain cancer'
December 12 2025 2:09 PM
The Democratic candidate in the Texas Senate race is going to be an LGBTQ+ ally
December 12 2025 12:55 PM
Texas expands lawsuits against doctors accused of providing gender-affirming care to youth
December 11 2025 4:36 PM
How Sundance 2026 celebrates its queer legacy
December 11 2025 3:54 PM
George Santos’s exclusive D.C. Christmas party featured famous grifters & MAGA influence peddlers
December 11 2025 3:31 PM
Nancy Mace investigated for bad behavior at airport, blames transgender people
December 11 2025 1:11 PM
Pete Buttigieg mocks Trump Transportation Secretary Sean Duffy’s strange airport pull-up stunt
December 11 2025 1:00 PM
Appeals court mulls upholding ruling that struck down Pentagon’s HIV enlistment ban
December 11 2025 11:51 AM
Florida sues leading medical groups for supporting gender-affirming care
December 11 2025 11:02 AM
Behind Marjorie Taylor Greene's latest push to criminalize gender-affirming care
December 10 2025 9:09 PM
Queer actor Wenne Alton Davis, known for 'Maisel,' 'Normal Heart,' killed in NYC car crash
December 10 2025 5:14 PM
‘Proud’ pro-LGBTQ+ Democrat flips Republican state House seat in Georgia electoral upset
December 10 2025 4:05 PM
Texas city votes to overturn LGBTQ+ antidiscrimination protections
December 10 2025 4:03 PM
Pornhub's spicy stats prove just how horny 2025 was
December 10 2025 3:30 PM
'Heated Rivalry' stars thank WeHo gay bar for 'tweeting about our butts'
December 10 2025 2:55 PM
Trending stories
Recommended Stories for You










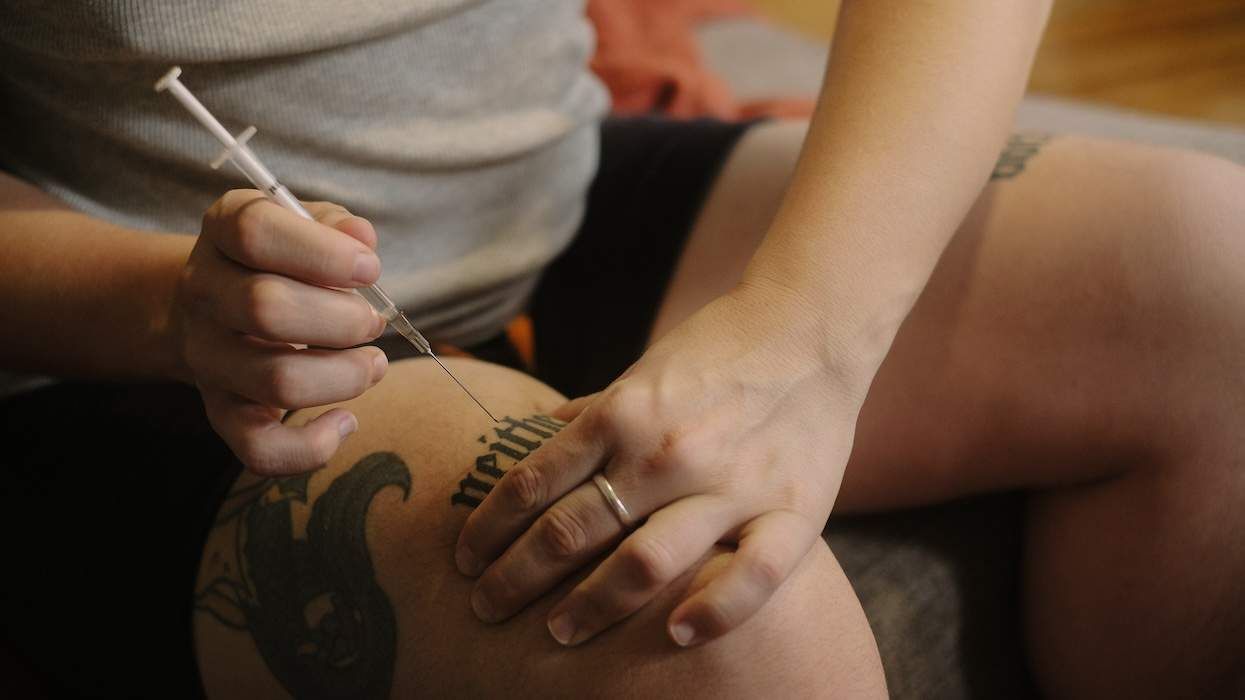

























































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes