November 04 2005 3:33 PM EST
CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
An HIV test that can be used at home and promises results in 20 minutes could help more people get treated sooner but raises concerns about how well patients could cope with the test findings on their own, a Food and Drug Administration advisory panel heard on Thursday in Gaithersburg, Md. Testing kits that allow consumers to mail a blood sample to a laboratory for results have been approved in the past, but advocates said allowing people to find out their HIV status at home makes testing easier, faster, and more private.
"A number of people don't get tested because they are concerned about privacy," Orasure Technology Inc. chief executive Douglas Michels told Reuters at the Food and Drug Administration panel meeting. The company is considering seeking FDA approval for a home version of its OraQuick test, which is already marketed to doctors and other health care providers.
No company has sought U.S. permission to sell such products over the counter, but the FDA is asking for the panelists' advice as it weighs how to review future proposals. Agency officials said there were several concerns about at-home HIV tests, including how people would cope with their results, especially those younger than 18. "Concerns have been expressed over the years about the psychological effects of receiving a positive HIV test result without the benefit of counseling. The issue that has come up repeatedly is suicidal tendencies," said Elliot Cowan, head of product reviews for the FDA's Division of Emerging and Transfusion Transmitted Diseases.
The FDA has been grappling with possible home-use HIV tests since 1986, when manufacturers first expressed interest in selling mail-in kits, which have since been approved for HIV and hepatitis C. OraQuick would allow consumers to insert a swab of saliva into a small bottle, providing the results while the patient waits at home. Like home pregnancy tests, various colored lines appear on a small window depending on whether the virus was detected. (Reuters)
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
Is Texas using driver's license data to track transgender residents?
December 15 2025 6:46 PM
Rachel Maddow on standing up to government lies and her Walter Cronkite Award
December 15 2025 3:53 PM
Beloved gay 'General Hospital' star Anthony Geary dies at age 78
December 15 2025 2:07 PM
Rob Reiner deserves a place in queer TV history for Mike 'Meathead' Stivic in 'All in the Family'
December 15 2025 1:30 PM
Culver City elects first out gay mayor — and Elphaba helped celebrate
December 15 2025 1:08 PM
Texas city cancels 2026 Pride after local council rescinds LGBTQ+ protections
December 15 2025 12:55 PM
North Carolina county dissolves library board for refusing to toss book about a trans kid
December 15 2025 11:45 AM
Florida and Texas launch 'legal attack' in push to restrict abortion medication nationally
December 15 2025 11:18 AM
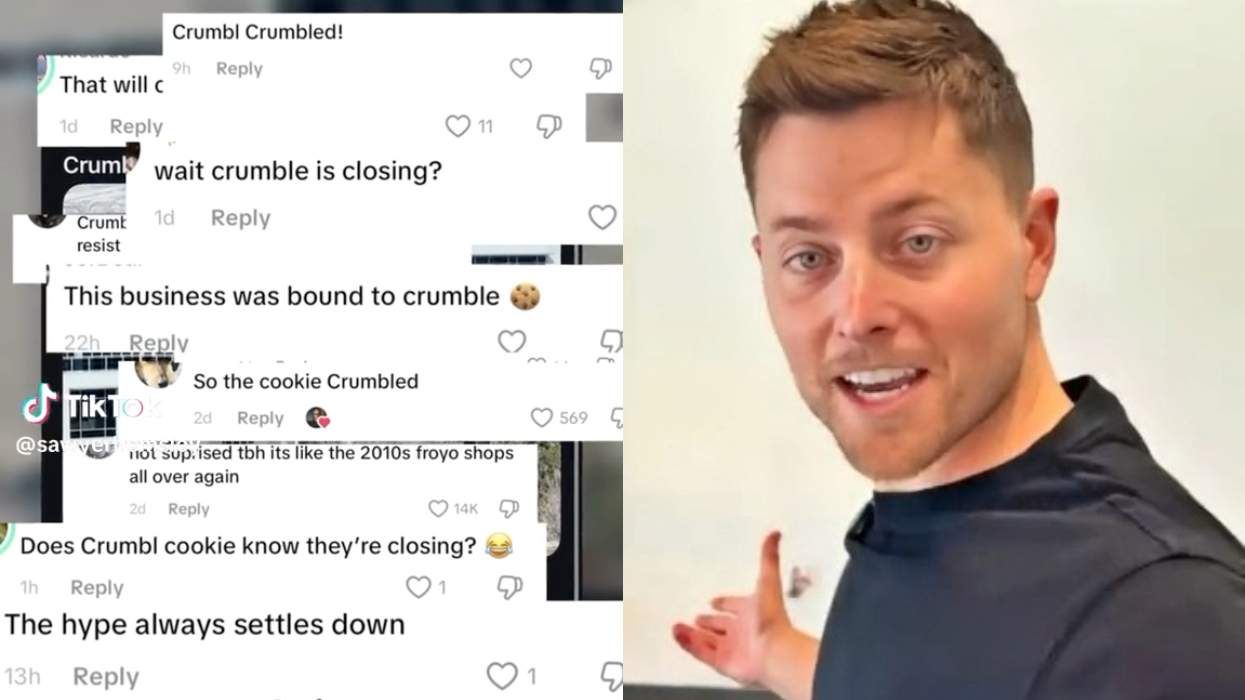
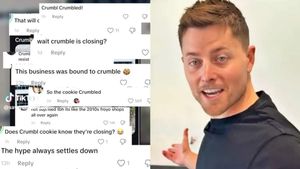
No, Crumbl is not Crumbl-ing, gay CEO Sawyer Hemsley says
December 15 2025 10:12 AM
11 times Donald Trump has randomly brought up his ‘transgender for everybody’ obsession
December 15 2025 9:22 AM
The story queer survivors aren't allowed to tell
December 15 2025 6:00 AM
Rob Reiner, filmmaker and marriage equality advocate, and wife Michele dead in apparent homicide
December 15 2025 1:08 AM
Trending stories
Recommended Stories for You
































































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes