CONTACTAbout UsCAREER OPPORTUNITIESADVERTISE WITH USPRIVACY POLICYPRIVACY PREFERENCESTERMS OF USELEGAL NOTICE
© 2025 Equal Entertainment LLC.
All Rights reserved
All Rights reserved
By continuing to use our site, you agree to our Privacy Policy and Terms of Use.
We need your help
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
Your support makes The Advocate's original LGBTQ+ reporting possible. Become a member today to help us continue this work.
The Food and Drug Administration has approved a new drug for the treatment of chronic hepatitis B, the drug's manufacturer said Wednesday. The drug, entecavir, is taken orally and is designed to work by preventing the virus that causes the illness from reproducing. The chronic form of hepatitis B can permanently damage the liver and lead to cirrhosis and liver cancer. Entecavir is made by Bristol-Myers Squibb and will be sold under the trade name Baraclude. The company said it could be available in early April. Current treatment options for chronic hepatitis include interferon, given by injection, and two drugs administered orally, lamivudine and adefovir dipivoxil. In clinical trials the main side effects reported for the new drug were headache, tiredness, dizziness, and nausea. The Hepatitis B Foundation estimates that 1.2 million Americans have hepatitis B and another 100,000 are infected annually. The hepatitis B virus can be transmitted through sexual activity, particularly through oral-anal contact. The Gay and Lesbian Medical Association urges all sexually active gay and bisexual men to be vaccinated against hepatitis A and B, but fewer than half of the nation's gay men have received the vaccines. (AP, with additional reporting by Advocate.com)
From our Sponsors
Most Popular
Bizarre Epstein files reference to Trump, Putin, and oral sex with ‘Bubba’ draws scrutiny in Congress
November 14 2025 4:08 PM
True
Jeffrey Epstein’s brother says the ‘Bubba’ mentioned in Trump oral sex email is not Bill Clinton
November 16 2025 9:15 AM
True
Watch Now: Pride Today
Latest Stories
Tucker Carlson and Milo Yiannopoulos spend two hours spewing homophobia and pseudo-science
December 04 2025 4:47 PM
'The Abandons' stars Gillian Anderson & Lena Headey want to make lesbian fans proud
December 04 2025 4:38 PM
Tig Notaro is working on a 'hot lesbian action' movie with Zack Snyder
December 04 2025 4:36 PM
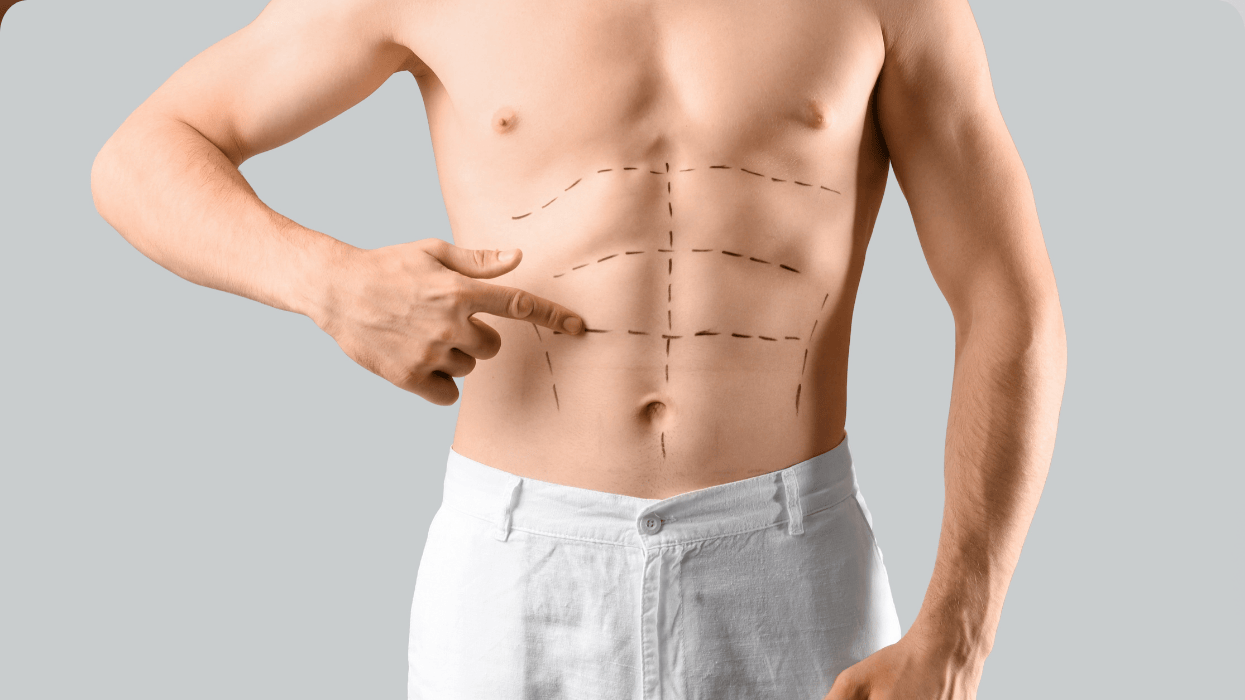
Cis men love top surgery—it should be available for all
December 04 2025 4:35 PM
Denver LGBTQ+ youth center closed indefinitely after burglar steals nearly $10K
December 04 2025 12:57 PM
Trans pastor says she’s ‘surrounded by loving kindness’ after coming out to New York congregation
December 04 2025 11:13 AM
Lesbian educator wins $700K after she was allegedly called a ‘witch’ in an ‘LGBTQ coven’
December 04 2025 10:59 AM
Years before Stonewall, a cafeteria riot became a breakthrough for trans rights
December 04 2025 10:50 AM
Charlie Kirk’s widow set to join out CBS News chief Bari Weiss for televised town hall
December 04 2025 10:20 AM
Women's Institute to ban transgender women after U.K. Supreme Court ruling
December 03 2025 4:10 PM
Trending stories
Recommended Stories for You
































































Charlie Kirk DID say stoning gay people was the 'perfect law' — and these other heinous quotes